Registration Requirements for Domestic Class II and III Medical Devices:
Required information:
1. Application form
2. Supporting documents
3. List of basic requirements for the safety and effectiveness of medical devices
4. Overview documents
5. Research documents
6. Manufacturing documents
7. Clinical evaluation documents
8. Product registration test report
9. Product technical requirements
10. Product registration test report
11. Sample instructions and labels
12. Declaration of conformity
Specific contents:
4.1 Overview
4.2 Product description
4.3 Model specifications
4.4 Packaging instructions
4.5 Scope of application and contraindications
4.6 Information on reference to similar/preceding products
4.7 Other elements to be described
5.1 Product performance studies
5.2 Bio-compatibility evaluation study
5.3 Bio-safety studies
5.4 Sterilization and disinfection process studies
5.5 Efficacy and packaging studies
5.6 Animal studies
5.7 Software studies
5.8 Other
6.1 Description of passive/active product production process information
6.2 Production sites
10.1 Registration inspection reports
10.2 Pre-evaluation comments
11.1 Instruction manual
11.2 Sample label for the smallest sales unit
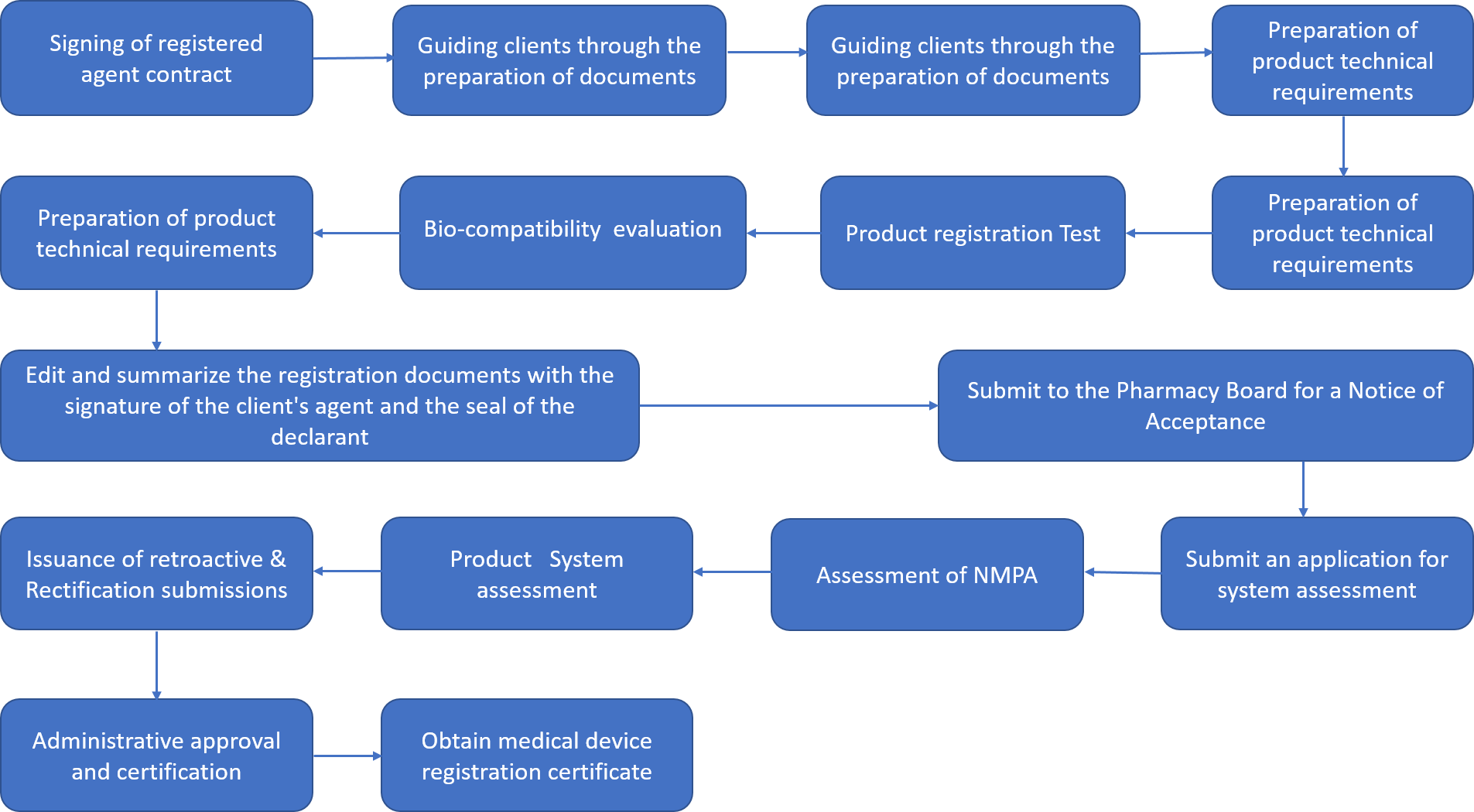
Registration processes of Class II and III Domestic Medical Device: